Abstract
Introduction Patients with peripheral T cell lymphoma (PTCL) lack good treatment options, particularly in the relapsed and refractory setting (Mak V et al. J Clin Oncol 2013). The development of the targeted therapies in PTCL has been lagging behind those developed for B cell lymphomas. Our work suggested that combination of epigenetic therapies could be a safe and effective approach in PTCL, particularly in T cell lymphomas with follicular helper phenotype (Marchi E et al. Br. J Haematol 2015; O'Connor AO et al; Blood 2019; Falchi L et al. Blood 2020). This could be explained by the presence of recurrent mutations in epigenetic factors, including Ten-Eleven Translocation-2 (TET2), DNA methyl transferase-3A (DNMT3A), and isocitrate dehydrogenase-2 (IDH2). that specifically clusters in PTCL (Couronné L. et al. N Eng J Med 2012; Lemonnier F et al. Blood 2012). Despite these observations, a direct link between presence of the mutations and sensitivity to treatment remain to be established.
Objectives To evaluate the merits of romidepsin plus azacitidine in patients with PTCL when administered in a 'real-world’ scenario (both subcutaneous and oral azacytidine). Our phase 2 study of oral azacitidine in combination with romidepsin showed remarkable results and we wanted to evaluate the efficacy of these two drugs in patients who were not enrolled on a clinical study for following reasons: 1) Rarity of PTCL prevents wide availability of clinical studies for this patient population; 2) Enrollment criteria, including performance status, prevent enrollment of all patients who are too frail but could possibly benefit from this treatment; 3) Second synchronous or recent malignancies.
Methods We retrospectively identified PTCL patients that were treated with azacitidine and romidepsin outside of a clinical trial based upon queries regarding off study use. The study was reviewed and approved by each Medical Center Institutional Review Board. We have identified 26 patients world-wide and these patients were treated using 4 different schedules: Schedule A: azacitidine 75mg/m2 s.c. on days 1-7, romidepsin 14 mg/m2 on day 1, 8 and 15 of a 28 day cycle (total of 7 patients); Schedule B: azacitidine 75mg/m2 s.c. on days 1-5, romidepsin 14 mg/m2 on day 8, 15 and 22 of a 35 day cycle; Schedule C (6 patients): azacitidine 75mg/m2 s.c. on days 1-7, romidepsin 12-14 mg/m2 on day 8, 15 and 22 of a 28 day cycle (6 patients); and Schedule D (as per Falchi L. et al. Blood 2020, 7 patients).
Results In our retrospective analysis we have identified 18 patients who had angioimmunoblastic lymphoma (AITL), 2 with adult T-cell leukemia/lymphoma (ATLL), 3 with TFH PTCL, 1 with PTCL-NOS and 2 with composite lymphoma (TFH PTCL with diffuse large B cell lymphoma and ALK- ALCL with FL).
Twelve of the 26 patients had next generation sequencing performed. Most common mutations found were those of TET2 (8 pts), RHOA (4pts), IDH2 (3pts) and DNMT3A (2 pts).
Among these 26 patients, overall response rate (ORR) was 73% and the complete response rate (CR) was 53%. Median number of cycles was 3.5 (range 1-12). Treatment was well tolerated and notable adverse effects included nausea, fatigue, rash, neutropenia and thrombocytopenia. One patient experienced febrile neutropenia while another had pulmonary infiltrates (drug toxicity vs. infection). Thrombocytopenia was the most common reason for dose reduction of romidepsin (to 12mg/m2) or its omission on day 8, 15 or 22.
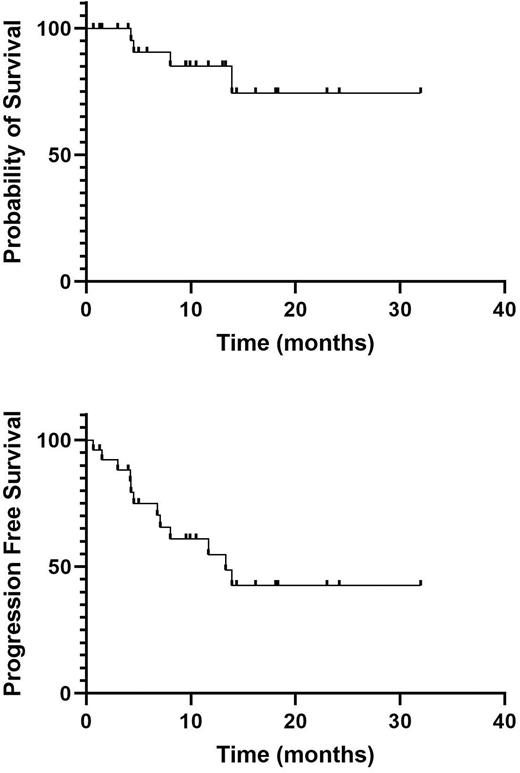
Median OS and PFS were not reached and 13.3 months respectively (Figure 1). Median DOR was not reached.
In 8 patients, azacitidine and romidepsin were used to achieve remission prior to stem cell transplant (1 auto, 7 allo), with all 7 patients were in CR at their last disease assessment. One patient died of transplant related mortality 8 months after his allogeneic stem cell transplant.
Conclusion Subcutaneous azacitidine and romidepsin administered in a 'real-world’ situation is highly effective in patients with relapsed PTCL with tolerable toxicity, and can be used to successfully bridge patients to stem cell transplant and to treat frail patients that would not be candidate for enrollment in clinical trials.. Notably, the efficacy was similar to the one reported on a clinical study with oral azacitidine and romidepsin. A randomized phase 2B trial of romidepsin and oral azacitidine versus investigator's choice in patients with R/R PTCL is currently ongoing and open to enrollment (NCT04747236).
Disclosures
Jain:Abcuro, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Acrotech LLC: Research Funding; Crispr Therapeutics: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees, Research Funding; Mersana Therapeutics: Research Funding; Myeloid Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; SecuraBio: Membership on an entity's Board of Directors or advisory committees; SIRPant Immunotherapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Tam:Janssen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Beigene: Honoraria, Research Funding; LOXO: Honoraria; AstraZeneca: Honoraria. O'Connor:TG Therapeutics: Current Employment, Current equity holder in publicly-traded company. Marchi:Myeloid Therapeutics: Ended employment in the past 24 months, Membership on an entity's Board of Directors or advisory committees; University of virginia: Patents & Royalties: 3062/170 PROV; Merck: Research Funding; Celgene/BMS: Research Funding; Astex Pharmaceuticals: Research Funding; NomoCan Pharmaceuticals: Research Funding; Kyowa Kirin: Honoraria; Daiichi Sankyo: Other: Participation at advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal